NUTRITERRA® DEVELOPMENT AND RESEARCH
A once-in-a-generation advancement like Nutriterra takes years to go from ideation to market. Here’s an overview of the science that went into developing and validating the world’s first plant-based source of total omega-3 nutrition.
Download our WhitepaperResearch Timeline
The Biotechnology… DHA Canola
At the dawn of the new millennium, it became clear that fish oil alone would not be able to meet the rising demand for omega-3 oil. To secure availability of these vital nutrients, researchers at Commonwealth Scientific and Industrial Research Organisation (CSIRO) and Grains Research Development Council (GRDC) and Nuseed applied biotechnology to develop a new source, DHA canola.
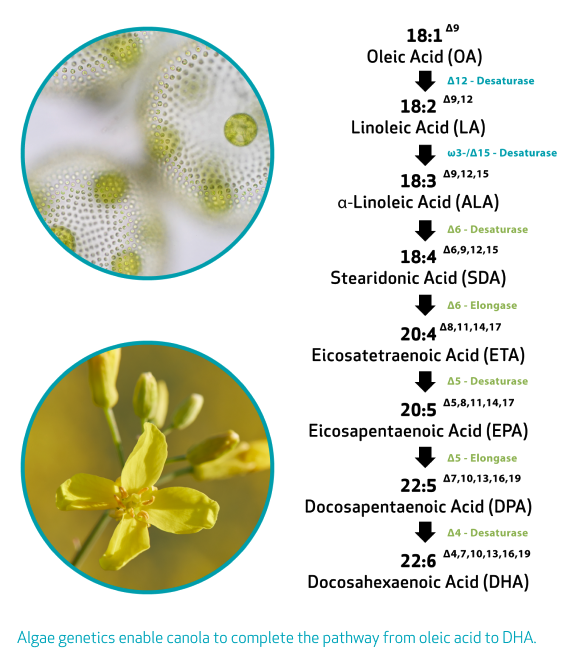
Brassica napus, or canola, was selected because it provides a highly scalable platform for commercially sustainable production. Canola is one of the world’s most important oilseed crops with production and infrastructure on five continents. Canola was also selected for its oil profile, which had the potential to produce long-chain polyunsaturated fatty acids (LCPUFA) from the oleic acid inherent to the crop.
The research team introduced omega-3 producing genes from microalgae and yeast to create an enzymatic pathway for canola to produce LCPUFAs, successfully developing the world’s first crop-based source of DHA and EPA.
Interested in the details of our biotech feat? Click the following link for the Petrie et al, 2020 publication.
Agronomy, Safety & Regulatory Studies
Approvals for Cultivation…
Field testing confirmed genetic and agronomic viability of DHA canola. Regulatory approvals for cultivation were achieved in Australia, USA, and Canada, leading to commercial production of seed oil for human and animal consumption.
…And for Consumption
Studies, described by Macintosh and colleagues, 2021 were conducted showing that, apart from the intended addition of LCPUFAs, omega-3 canola is compositionally similar to conventional canola. This study, as well as another described by Murillo and colleagues, 2021 supported regulatory approval for human and animal use in Australia, Canada, and USA.
This research was included in our submissions to the US Food and Drug Administration (FDA) and supported their recognition of Nutriterra® as a New Dietary Ingredient (NDI) and Generally Recognized As Safe (GRAS). Additional global regulatory evaluations are ongoing.
Nutriterra efficacy demonstrated in Clinical Trial
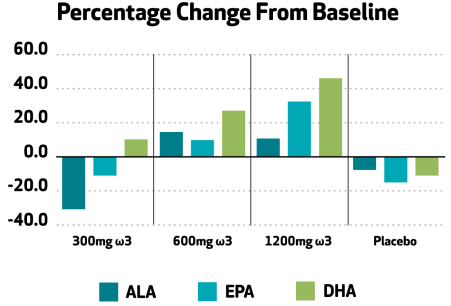
Lin and colleagues, 2022 conducted Phase I and II clinical research to evaluate three levels of Nutriterra intake in pharmacokinetic (PK) and efficacy studies. The PK study showed that the fatty acids in Nutriterra were bioavailable (absorbed into the body), while the efficacy study showed significantly improved short and long-term LCPUFA profiles and improvements in indicators of O3 status. What’s more, the important ω6:ω3 ratio was improved.
Nutriterra improves Omega-3 status
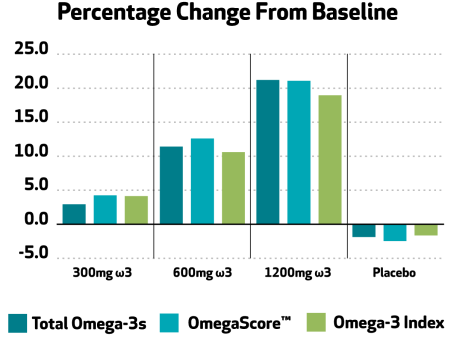
Omega-3 status was measured three ways in this clinical study.
- Total Omega-3 levels
- Omega-Score
- Omega-3 Index
Each of these indices were shown to be increased by consumption of Nutriterra, demonstrating efficacy of this novel omega-3 source in improving omega-3 status.
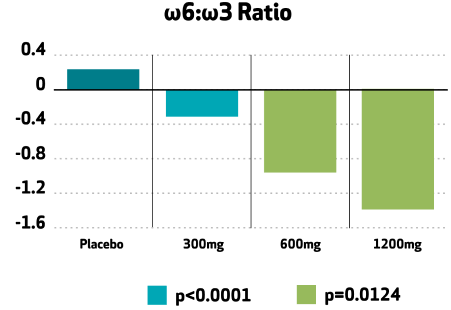
Plus, Nutriterra supports a healthy ω6:ω3 ratio
Evaluation of the relationship between Nutriterra intake and the omega 6:omega 3 in this clinical showed that with increasing levels of Nutriterra intake, the ratio improved. In the graph below a negative ratio change means the levels of omega-6 are reduced, which has been shown to be beneficial.
Download our Whitepaper
Nutriterra References
Petrie JR, Zhou X-R, Leonforte A, Mcallister J, et al.
- 2020 – Development of a Brassica napus (canola) crop containing fish oil-like levels of DHA in the seed oil. Frontiers in Plant Science 11, 727.
Macintosh SC, Shaw M, Connelly M, Yao ZJ.
- 2021 – Food and feed safety of NS-B5ØØ27-4 omega-3 canola (Brassica napus): A new source of long-chain omega-3 fatty acids. Frontiers in Nutrition 8: 716659.
Murillo G, Horn T, Johnson WD, MacIntosh S.
- 2021 – 28-Day oral (gavage) and 13-week (dietary) toxicity studies of DHA canola oil and DHA canola meal in rats, Regulatory Toxicology and Pharmacology. 127,105050.
Lin XL, Baisley J, Bier A, Vora D, Holub B.
- Blood omega-3 profiles were significantly improved after single and multiple doses of a novel transgenic canola oil containing long-chain polyunsaturated omega-3 fat:y acids: A randomized, placebo-controlled trial in healthy adults. Frontiers in Nutrition 9:847114.
These statements have not been evaluated by the Food and Drug Administration.
This product is not intended to diagnose, treat, cure, or prevent any disease.